Video - We are the Second Line of Defense
To watch the video with subtitles, choose your language: PT | CN | EN
English |
SARS-CoV-2: What & How
Question: What is a virus?
Answer: A virus is not a bacteria. A virus is a microorganism that causes damage in the body by invading cells (these cells are called hosts). Unlike bacteria, all viruses cause disease.
Question: What is the difference between a virus and a bacterium?
Answer: Both bacteria and viruses are microorganisms. But think of it this way: an elephant and a rat are completely different but they’re both animals. Same goes for the virus and bacteria.
Additionally, one of the biggest differences between bacteria and viruses, is their size. A bacterium is the size of a large orange, in comparison to a virus which would be the size of an apple seed.
Question: How is Covid-19 transmitted?
Answer: The main route of transmission is through respiratory droplets. We expel these when we sneeze, cough or even when we talk! Another common route includes touching surfaces that have the virus and then touching your face (before washing your hands).
Find more in this article published in Elsevier
Question: Can a virus live on non-living surfaces?
Answer: Yes. Although viruses live off of other organisms, such as animals or plants, they can be found on non-living surfaces. However, a virus cannot survive for a very long time on non-living surfaces.
Find more on The New England Journal of Medicine
Question: How long does the virus last on surfaces?
Answer:
- Food: no evidence to suggest it spreads through food
- Paper: from a few minutes up to 5 days
- Cardboard: ~ 24 hours
- Wood: ~ 4 days
- Plastic: ~ 2/3 days
- Metals: ~ 5 days
- Steel: ~ 2/3 days
The overall survival rate of the virus on surfaces may vary slightly according to the amount of sunlight and humidity the virus gets exposed to.
Find more on The New England Journal of Medicine
Question: Will warm weather stop the outbreak of Covid-19?
Answer: It is not yet known whether weather and temperature affect the spread of Covid-19. Some other viruses, like those that cause the common cold and flu, spread more during cold weather months but that does not mean it is impossible to become sick with these viruses during other months. There is much more to learn about the transmissibility, severity, and other features associated with Covid-19 and investigations are ongoing.
Question: Can someone who has been quarantined for Covid-19 spread the illness to others?
Answer: Quarantine means separating a person or group of people who have been exposed to a contagious disease but have not developed illness (symptoms) from others who have not been exposed, in order to prevent the possible spread of that disease. Quarantine is usually established for the incubation period of the communicable disease, which is the span of time during which people have developed illness after exposure. For Covid-19, the period of quarantine is 14 days from the last date of exposure because the incubation period for this virus is 2 to 14 days. Someone who has been released from Covid-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Question: How does Covid-19 affect our cells?
Answer: Covid-19 is particularly good at sticking to respiratory tissue. This means attaching to cells found mainly in the lungs. The virus does this by using its crown-like spikes which are attached to the virus’ envelope. As a result, one virus particle can attach itself to multiple cells in the body.
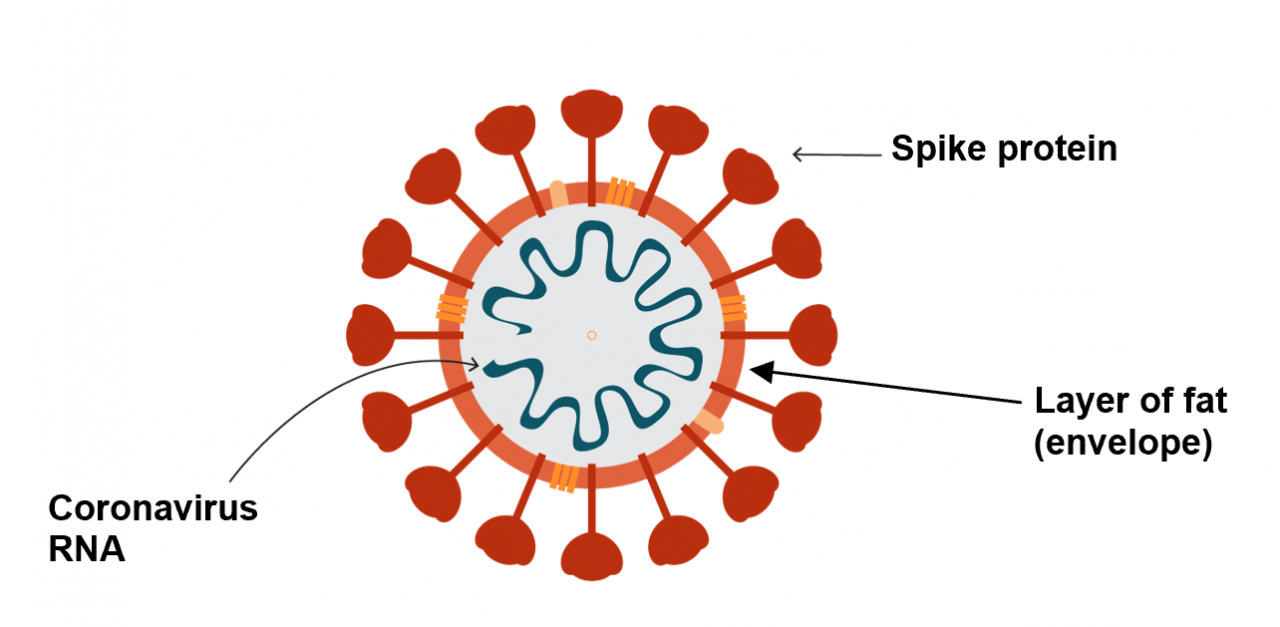
The easiest paths of entry for the virus are the mouth, nose and eyes. Once the virus manages to attach itself to the host cell, the host cell will take the virus particle in. The virus will then release genetic information called RNA. RNA acts as a set of instructions that tells the host cell what to do. Therefore, RNA will stop a cell from functioning normally, and instead will convert the healthy host cell into a virus making factory. Once the new virus is assembled inside the host cell, it is released into the surroundings, hence causing even further infection in the body.
If our immune system is not effective at fighting the virus and stopping it from spreading onto further cells, more virus-making factories are created in our body using our cells. This eventually kills the host cell, as it is starved by the virus, causing us to have severe respiratory problems, amongst other consequences.
Question: Why do we need to use facemasks?
Answer: Facemasks reduce the distance respiratory particles can travel. This means that it reduces the chances of getting infected by preventing the spread of respiratory droplets. It also acts as a filter to the air you breath in and so prevents you from inhaling the virus.
Question: How does washing our hands with soap, get rid of the virus?
Answer: Soap is crucial as it contains chemicals that break down the layer of fat surrounding the coronavirus (envelope) and hence completely destroy it. Using only water doesn’t help because it doesn’t stop the virus from sticking to your hand.
Watch this video to learn how soap kills the coronavirus
Question: Why should you avoid touching your face?
Answer: This is because the virus can easily enter the body through the mouth, nose or even eyes. However, the virus doesn’t enter your body through the skin, as this acts as a physical barrier.
Question: Should I take antibiotics to treat Covid-19 and are anti-bacterial products useful?
Answer: Antibiotics are not designed to stop viral infections and therefore are of no use when trying to treat Covid-19. As explained above, bacteria and viruses are two very different microorganisms. This means that anti-bacterial products are not useful either, for when disinfecting surfaces from Covid-19. Instead you should use disinfectant or alcohol.
Question: Is the virus’ ability to spread affected by the climate?
Answer: So far there is no scientific evidence to suggest or prove that the virus is less effective at spreading in hot or humid weather.
Question: What are the symptoms of Covid-19?
Answer: People with Covid-19 have had a wide range of symptoms reported – ranging from mild symptoms to severe illness. Symptoms may appear 2-14 days after exposure to the virus. People with these symptoms may have Covid-19:
- Fever or chills
- Cough
- Shortness of breath or difficulty breathing
- Fatigue
- Muscle or body aches
- Headache
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
- Diarrhea
This list does not include all possible symptoms. CDC will continue to update this list as we learn more about Covid-19.
Question: Can someone test negative and later positive on a viral test for Covid-19?
Answer: Yes, it is possible. You may test negative if the sample was collected early in your infection and test positive later during this illness. You could also be exposed immune to Covid-19 after the test and get infected then. Even if you test negative, you still should take steps to protect yourself and others. See Testing for Current Infection for more information.
Question: If I have recovered from Covid-19, will I be immune to it?
Answer: We do not know yet if people who recover from Covid-19 can get infected again. CDC and partners are investigating to determine if a person can get sick with Covid-19 more than once. Until we know more, continue to take steps to protect yourself and others.
Question: Who is at higher risk for serious illness from Covid-19?
Answer: COVID-19 is a new disease and there is limited information regarding risk factors for severe disease. Based on currently available information and clinical expertise, older adults and people of any age who have serious underlying medical conditions might be at higher risk for severe illness from Covid-19.
Based on what we know now, those at high-risk for severe illness from Covid-19 are:
- People aged 65 years and older
- People who live in a nursing home or long-term care facility
- People of all ages with underlying medical conditions, particularly if not well controlled, including:
- People with chronic lung disease or moderate to severe asthma
- People who have serious heart conditions
- People who are immunocompromised
Many conditions can cause a person to be immunocompromised, including cancer treatment, smoking, bone marrow or organ transplantation, immune deficiencies, poorly controlled HIV or AIDS, and prolonged use of corticosteroids and other immune weakening medications
- People with severe obesity (body mass index [BMI] ≥40)
- People with diabetes
- People with chronic kidney disease undergoing dialysis
- People with liver disease
Question: What should people at higher risk of serious illness with Covid-19 do?
Answer: If you are at higher risk of getting very sick from Covid-19, you should:
- Stock up on supplies
- Take everyday precautions to keep space between yourself and others
- When you go out in public, keep away from others who are sick
- Limit close contact and wash your hands often
- Avoid crowds, cruise travel, and non-essential travel
If there is an outbreak in your community, stay home as much as possible. Watch for symptoms and emergency signs. If you get sick, stay home and call your doctor. More information on how to prepare, what to do if you get sick, and how communities and caregivers can support those at higher risk is available on People at Risk for Serious Illness from COVID-19.
Useful links
- httpss://www.youtube.com/watch?v=O-3Mlj3MQ_Q&feature=youtu.be
- httpss://www.youtube.com/watch?v=n6QwnzbRUyA&feature=youtu.be
- httpss://www.livescience.com/coronavirus-kids-guide.html
- httpss://www.natgeokids.com/au/discover/science/general-science/what-is-coronavirus/
- httpss://www.youtube.com/watch?v=BE-cA4UK07c&feature=youtu.be
- httpss://www.journalofhospitalinfection.com/article/S0195-6701(20)30046-3/fulltext
- httpss://www.wjgnet.com/2307-8960/full/v8/i8/1391.htm
Relevant Information
| Hovione Practical Handbook - Covid-19 |
| The Coronavirus Prevention Handbook |
| Spanish Flu book chapter |
|
Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Provisional 6th Edition) |
|
WHO-2019-nCoV-IPC - Water, sanitation, hygiene, and waste management for the COVID-19 virus |
| WHO-recommended Handrub Formulations |
Rate of growth of COVID-19
| SARS-Cov-2 Report - 30 November 2021 |
Hovione Crisis Response
| COVID-19 - Hovione Crisis Response |
| COVID-19 Preparedness Response - Countermeasures adopted prior to Chinese New Year at the Hovione Macau plant |
Stakeholders Communiqués
| Communication of Site Shutdown - Hovione Macau | 15 July 2022 |
| 16th Stakeholder Communiqué Covid-19 | 11 May 2021 |
| 15th Stakeholder Communiqué Covid-19 | 12 March 2021 |
| 14th Stakeholder Communiqué Covid-19 | 5 February 2021 |
| 13th Stakeholder Communiqué Covid-19 | 12 January 2021 |
| 12th Stakeholder Communiqué Covid-19 | 7 December 2020 |
| 11th Stakeholder Communiqué Covid-19 | 5 November 2020 |
| 10th Stakeholder Communiqué Covid-19 | 8 October 2020 |
| 9th Stakeholder Communiqué - Covid-19 | 2 September 2020 |
| 8th Stakeholder Communiqué - Covid-19 | 12 August 2020 |
| 7th External Stakeholder Communiqué - Covid-19 | 9 July 2020 |
| 6th External Stakeholder Communiqué - Covid-19 | 8 June 2020 |
| 5th External Stakeholder Communiqué - Covid-19 | 6 May 2020 |
| Covid-19 FAQ | 20 April 2020 |
| 4th External Stakeholder Communiqué - Covid-19 | 3 April 2020 |
| 20 March 2020 | |
| 13 March 2020 | |
| 12 February 2020 | |
| 31 January 2020 |
Webinar
| Webinar Presentation Sharing our Covid-19 experience. Seeking Feedback |
14 July 2020 |
Service Providers and External Visitors
| Guidance Protocol for External Visitors |
Contingency Plan - Covid-19
Videos
We are the Second Line of Defense (27 April 2020)
To watch the video with subtitles, choose your language: PT | CN | EN
Message from Élie Vannier, Chairman of the Board of Directors (30 July 2020)
Language: English and subtitles in Portuguese
Return From Home Rules (7 July 2020)
Message from Filipe Gaspar, Chief Technology Officer (7 April 2020)
Language: Portuguese and subtitles in English
Message from Tiago Ferreira de Matos, General Counsel (31 March 2020)
Language: Portuguese and subtitles in English
Guy Villax message - Washing hands (25 March 2020)
Message from Eddy Leong, Site Manager - Macao (23 March 2020)
What can people do to protect themselves and others from getting the new coronavirus
Source: WHO, World Health Organization
WHO: How to handwash? With soap and water
Source: WHO, World Health Organization
Can masks protect against the new coronavirus infection?
Source: WHO, World Health Organization
Certifications

As a result of a joint effort between Hovione and Gertal, Sete Casas and Lumiar Canteens have been certified by APCER with the “Covid Safe Verified” seal.
“Covid Safe” is a brand developed by APCER that certifies compliance with the new health and safety rules, in the context of the Covid-19 pandemic. This certification is the recognition of the concern of both Hovione and Gertal in complying with the rules established and providing the best safety conditions for our Team Members.
ABC Covid-19 (for schools)
ABC Covid-19 initiative aims to help schools across the country in their return to classes in a practical, direct, and efficient way, by providing support to answer the various difficulties they face in their daily routines.
The team is composed of trainees and is supervised by the Covid-19 prevention team at Hovione and their aim is to inform students about what are good habits to adopt and how to prevent risks while also supporting teachers and staff with up-to-date, reliable and clear information.
Here you can find all the information provided in a poster format that you can distribute throughout the school. Portuguese only.
Follow us:

|

|

|

|
17-year arts student wins ABCovid contestTomás Oliveira, aged 17, from Escola Artística António Arroio, with the video "Covid-19 simplified for morons", which explains simply and graphically why we need a mask to protect ourselves from this virus. The ABCovid contest is an initiative taken by Hovione trainees aimed at elementary and high school students. For more details visit www.abcovid.pt |
Português |
SARS-CoV-2: O Quê & Como
Pergunta: O que é um vírus?
Resposta: Um vírus não é uma bactéria. Um vírus é um microrganismo que causa danos ao organismo, invadindo as células (essas células são chamadas de hospedeiros). Ao contrário das bactérias, todos os vírus causam doenças.
Pergunta: Qual é a diferença entre um vírus e uma bactéria?
Resposta: Bactérias e vírus são microrganismos. Mas pense assim: um elefante e um rato são completamente diferentes, mas ambos são animais. O mesmo vale para o vírus e as bactérias.
Além disso, uma das maiores diferenças entre bactérias e vírus é o seu tamanho. Uma bactéria é do tamanho de uma laranja grande, em comparação com um vírus do tamanho de uma semente de maçã.
Pergunta: Como o Covid-19 é transmitido?
Resposta: A principal via de transmissão é através de gotículas respiratórias. Nós os expulsamos quando espirramos, tossimos ou mesmo quando conversamos! Outra rota comum inclui tocar em superfícies com o vírus e depois tocar em seu rosto (antes de lavar as mãos).
Saiba mais neste artigo científico publicado na Elsevier
Pergunta: Um vírus pode viver em superfícies não-vivas?
Resposta: Sim. Embora os vírus vivam de outros organismos, como animais ou plantas, eles podem ser encontrados em superfícies não-vivas. No entanto, um vírus não pode sobreviver por muito tempo em superfícies não-vivas.
Saiba mais neste artigo publicado na The New England Journal of Medicine
Pergunta: Quanto tempo dura o vírus nas superfícies?
Resposta:
- Comida: nenhuma evidência para sugerir que ela se espalha através da comida
- Papel: de alguns minutos a 5 dias
- Papelão: ~ 24 horas
- Madeira: ~ 4 dias
- Plástico: ~ 2/3 dias
- Metais: ~ 5 dias
- Aço: ~ 2/3 dias
A taxa geral de sobrevivência do vírus nas superfícies pode variar ligeiramente de acordo com a quantidade de luz solar e umidade à qual o vírus é exposto.
Saiba mais neste artigo publicado na The New England Journal of Medicine
Pergunta: O tempo quente vai parar o surto de Covid-19?
Resposta: Ainda não se sabe se o clima e a temperatura afetam a propagação do Covid-19. Alguns outros vírus, como os que causam o resfriado e a gripe comuns, se espalham mais durante os meses de clima frio, mas isso não significa que é impossível ficar doente com esses vírus durante outros meses. Há muito mais para aprender sobre a transmissibilidade, a gravidade e outros recursos associados ao Covid-19 e às investigações em andamento.
Pergunta: Alguém que foi colocado em quarentena por Covid-19 pode espalhar a doença para outras pessoas?
Resposta: Quarentena significa separar uma pessoa ou grupo de pessoas que foram expostas a uma doença contagiosa, mas não desenvolveram doenças (sintomas) de outras que não foram expostas, a fim de impedir a possível propagação dessa doença. A quarentena é geralmente estabelecida para o período de incubação da doença transmissível, que é o período durante o qual as pessoas desenvolvem doenças após a exposição. Para o COVID-19, o período de quarentena é de 14 dias a partir da última data de exposição, porque o período de incubação desse vírus é de 2 a 14 dias. Alguém que foi liberado da quarentena de COVID-19 não é considerado um risco de espalhar o vírus para outras pessoas porque não desenvolveram doenças durante o período de incubação.
Pergunta: Como o Covid-19 afeta as nossas células?
Resposta: O Covid-19 é particularmente bom em aderir ao tecido respiratório. Isso significa anexar às células encontradas principalmente nos pulmões. O vírus faz isso usando seus picos em forma de coroa, anexados ao envelope do vírus. Como resultado, uma partícula de vírus pode se conectar a várias células do corpo.
Os caminhos mais fáceis de entrada para o vírus são a boca, nariz e olhos. Uma vez que o vírus consiga se conectar à célula hospedeira, a célula hospedeira absorve a partícula do vírus. O vírus libera informações genéticas chamadas RNA. O RNA atua como um conjunto de instruções que informa à célula hospedeira o que fazer. Portanto, o RNA interrompe o funcionamento normal da célula e, em vez disso, converte a célula hospedeira saudável em uma fábrica de criação de vírus. Uma vez que o novo vírus é montado dentro da célula hospedeira, ele é liberado para o ambiente, causando ainda mais infecções no organismo.
Se nosso sistema imunológico não é eficaz no combate ao vírus e impede que ele se espalhe para outras células, mais fábricas de criação de vírus são criadas em nosso corpo, usando nossas células. Isso acaba matando a célula hospedeira, pois ela sofre de fome pelo vírus, causando problemas respiratórios graves, entre outras consequências.

Pergunta: Por que precisamos usar máscaras?
Resposta: As máscaras faciais reduzem a distância que as partículas respiratórias podem percorrer. Isso significa que reduz as chances de ser infectado, impedindo a propagação de gotículas respiratórias. Também atua como um filtro para o ar que você respira e impede a inalação do vírus.
Pergunta: Como lavar as mãos com sabão elimina o vírus?
Resposta: O sabão é crucial, pois contém produtos químicos que quebram a camada de gordura ao redor do coronavírus (envelope) e, portanto, o destroem completamente. Usar apenas água não ajuda, pois não impede que o vírus grude na sua mão.
Assista a este vídeo para saber como é que o sabão elimina o vírus
Pergunta: Por que você deve evitar tocar no seu rosto?
Resposta: Isso ocorre porque o vírus pode facilmente entrar no corpo pela boca, nariz ou até olhos. No entanto, o vírus não entra no seu corpo através da pele, pois atua como uma barreira física.
Pergunta: Devo tomar antibióticos para tratar o Covid-19 e os produtos antibacterianos são úteis?
Resposta: Os antibióticos não são projetados para interromper infecções virais e, portanto, não são úteis ao tentar tratar o Covid-19. Como explicado acima, bactérias e vírus são dois microorganismos muito diferentes. Isso significa que os produtos antibacterianos também não são úteis, ao desinfetar superfícies do Covid-19. Em vez disso, você deve usar desinfetante ou álcool.
Pergunta: A capacidade do vírus se espalhar é afetada pelo clima?
Resposta: Até o momento, não há evidências científicas para sugerir ou provar que o vírus é menos eficaz na propagação em clima quente ou úmido.
Pergunta: Quais são os sintomas da Covid-19?
Resposta: Pessoas com Covid-19 tiveram uma ampla gama de sintomas relatados - desde sintomas leves a doenças graves. Os sintomas podem aparecer 2-14 dias após a exposição ao vírus. Pessoas com esses sintomas podem ter Covid-19:
- Febre ou calafrios
- Tosse
- Falta de ar ou dificuldade em respirar
- Fadiga
- Dores musculares ou corporais
- Dor de cabeça
- Nova perda de paladar ou olfato
- Dor de garganta
- Congestão ou coriza
- Náusea ou vômito
- Diarreia
Esta lista não inclui todos os sintomas possíveis. O CDC continuará a atualizar esta lista à medida que aprendermos mais sobre o Covid-19.
Pergunta: Alguém pode testar negativo e posteriormente positivo num teste viral para o Covid-19?
Resposta: Sim, é possível. Você pode ter um resultado negativo se a amostra foi coletada no início de sua infeção e um resultado positivo mais tarde durante esta doença. Você também pode ser exposto ao Covid-19 imune após o teste e infetado. Mesmo se você testar negativo, você ainda deve tomar medidas para proteger a si e aos outros. Consulte Teste para infecção atual para obter mais informações.
Pergunta: Se eu me recuperei do Covid-19, estarei imune?
Resposta: Ainda não sabemos se as pessoas que se recuperam do Covid-19 de ficar doente com Covid-19 mais de uma vez. Até que saibamos mais, continue a tomar medidas para proteger a si e aos outros.
Pergunta: Quem está em maior risco de doenças graves por causa da Covid-19?
Resposta: Covid-19 é uma doença nova e há informações limitadas sobre fatores de risco para doenças graves. Com base nas informações disponíveis no momento e nos conhecimentos clínicos, idosos e pessoas de qualquer idade com sérias condições médicas subjacentes podem estar em maior risco de doença grave por causa do Covid-19.
Com base no que sabemos agora, aqueles com alto risco de doença grave por Covid-19 são:
- Pessoas com 65 anos ou mais
- Pessoas que vivem em um lar de idosos ou em instituições de longa permanência
- Pessoas de todas as idades com condições médicas subjacentes, principalmente se não forem bem controladas, incluindo:
- Pessoas com doença pulmonar crônica ou asma moderada a grave
- Pessoas que têm problemas cardíacos graves
- Pessoas imunológicas
Links Úteis
- httpss://www.youtube.com/watch?v=O-3Mlj3MQ_Q&feature=youtu.be
- httpss://www.youtube.com/watch?v=n6QwnzbRUyA&feature=youtu.be
- httpss://www.livescience.com/coronavirus-kids-guide.html
- httpss://www.natgeokids.com/au/discover/science/general-science/what-is-coronavirus/
- httpss://www.youtube.com/watch?v=BE-cA4UK07c&feature=youtu.be
- httpss://www.journalofhospitalinfection.com/article/S0195-6701(20)30046-3/fulltext
- httpss://www.wjgnet.com/2307-8960/full/v8/i8/1391.htm
Informação Relevante
| Manual Prático Colaborador Hovione - Covid-19 |
| Manual para a Prevenção e Tratamento da Covid-19 |
|
WHO-2019-nCoV-IPC - Water, sanitation, hygiene, and waste management for the COVID-19 virus |
Webinar
| Apresentação Webinar Resposta a Covid-19 - Partilha de experiências. Juntos somos mais fortes |
16 julho 2020 |
Prestadores de Serviços
Prestadores de Serviços: Plano de Contingência COVID-19
| Regras de Circulação em Instalações Hovione em Sete Casas e Lumiar |
Videos
Hovione - Messagem de Élie Vannier, Presidente do Conselho de Administração (30 de julho 2020)
Legendado em Português
ABCovid: Entrevista de Santiago Sampaio no programa Curto Circuito, SIC Radical (13 de julho 2020)
Saber mais sobre o Concurso Nacional ABCovid
Mensagem de Ana Cristina Guimarães, Diretora de RH Portugal (26 de junho de 2020)
Mensagem de Isabel Jonet, Banco Alimentar Contra a Fome (5 de junho 2020)
Regras de Regresso de Casa (28 de maio de 2020)
Regras de Regresso de Casa - Regra 1
Regras de Regresso de Casa - Regra 2
Regras de Regresso de Casa - Regra 3
Regras de Regresso de Casa - Regra 4 e 5
Regras de Regresso de Casa - Regra 6
Regras de Regresso de Casa - Regra 7
Regras de Regresso de Casa - Regra 8
Regras de Regresso de Casa - Regra 9
Regras de Regresso de Casa - Regra 10
Regras de Regresso de Casa - Regra 11
Histórias de Superação (1 de maio de 2020)
Somos a segunda linha de defesa (27 de abril de 2020)
Video disponível com legendas em CN | EN
Mensagem de Filipe Gaspar, Chief Technology Officer (7 de abril de 2020)
Mensagem de Tiago Ferreira de Matos, General Counsel (31 de março de 2020)
Mensagem de Guy Villax - Lavagem de mãos (25 de março de 2020)
Mensagem de Guy Villax a partir da fábrica de Sete Casas em Portugal (19 de março de 2020)
Medidas internas coronavírus com procedimentos à chegada aos nossos sites (6 de março de 2020)
Recomendação de medidas de proteção individual e coletiva na prevenção da infeção com COVID-19
Fonte: DGS, Direção-Geral da Saúde
Prevenção e Proteção
Fonte: DGS, Direção-Geral da Saúde
O novo coronavírus - COVID-19
Fonte: DGS, Direção-Geral da Saúde
Empresas alteram produção para fabricar desinfetantes
Fonte: RTP1

Certificações

Em resultado de um esforço conjunto entre a Hovione e a Gertal, as Cantinas de Sete Casas e do Lumiar foram certificadas pela APCER com o selo “Covid Safe Verified”.
“Covid Safe” é uma marca desenvolvida pela APCER que certifica o cumprimento das novas regras de segurança e saúde, no contexto da pandemia da Covid-19. Esta certificação é o reconhecimento da preocupação da Hovione e da Gertal em cumprir as regras estabelecidas e em proporcionar as melhores condições de segurança aos nossos Colaboradores.
Notícias / Media
| Inspirar à ação… para uma saúde de qualidade (ODS 3) | 29 janeiro 2021 |
ABC Covid-19 (para escolas)
A iniciativa ABC Covid-19 visa ajudar as escolas de todo o país com o regresso às aulas de uma forma prática, eficaz e direta, dando apoio para enfrentar as dificuldades encontradas no funcionamento diário.
A equipa, composta por estagiários e supervisionada pela equipa de resposta ao Covid-19 da Hovione, pretende ajudar a informar os alunos dos bons hábitos a adotar e como prevenir os riscos, mas também dar apoio aos professores e funcionários para que estes tenham acesso a informação fidedigna, atualizada e simples.
Aqui pode encontrar toda a nossa informação em formato PDF para poder utilizar em forma de posters na escola.
Segue-nos nas redes sociais:

|

|

|

|
Esta certificação tem a validade de 6 meses e é renovada após auditorias de acompanhamento previamente agendadas ou sem aviso prévio.
Aluno de artes de 17 anos da António Arroio vence concurso ABCovidTomás Oliveira, de 17 anos, aluno da Escola Artística António Arroio, em Lisboa, é o vencedor do concurso ABCovid, com o vídeo “Covid-19 simplificado para totós”, em que explica de modo simples e gráfico porque precisamos de máscara para nos protegermos do novo coronavírus. O concurso ABCovide é uma iniciativa dos estagiários da Hovione dirigida a estudantes do ensino básico e secundário. Para mais informações visite a página www.abcovid.pt |