API
Hovione inhalation grade APIs bring unique controlled performance to your formulation.
Focused expertise in Drug Substance manufacturing processes
Inhalation Drug substance services
Hovione has been developing and scaling tough chemistries for over sixty years and in that timeframe has assembled the necessary technical capabilities and synthetic experience which enable us to perform almost any chemical transformation. With decades of experience in developing and commercializing products from these foundations, Hovione provides strong partnership for all your Drug Substance challenges and needs.
Customized Inhalation & Nasal grade API
Hovione has developed a range of APIs with superior performance that address the challenges of orally inhaled and nasal products.
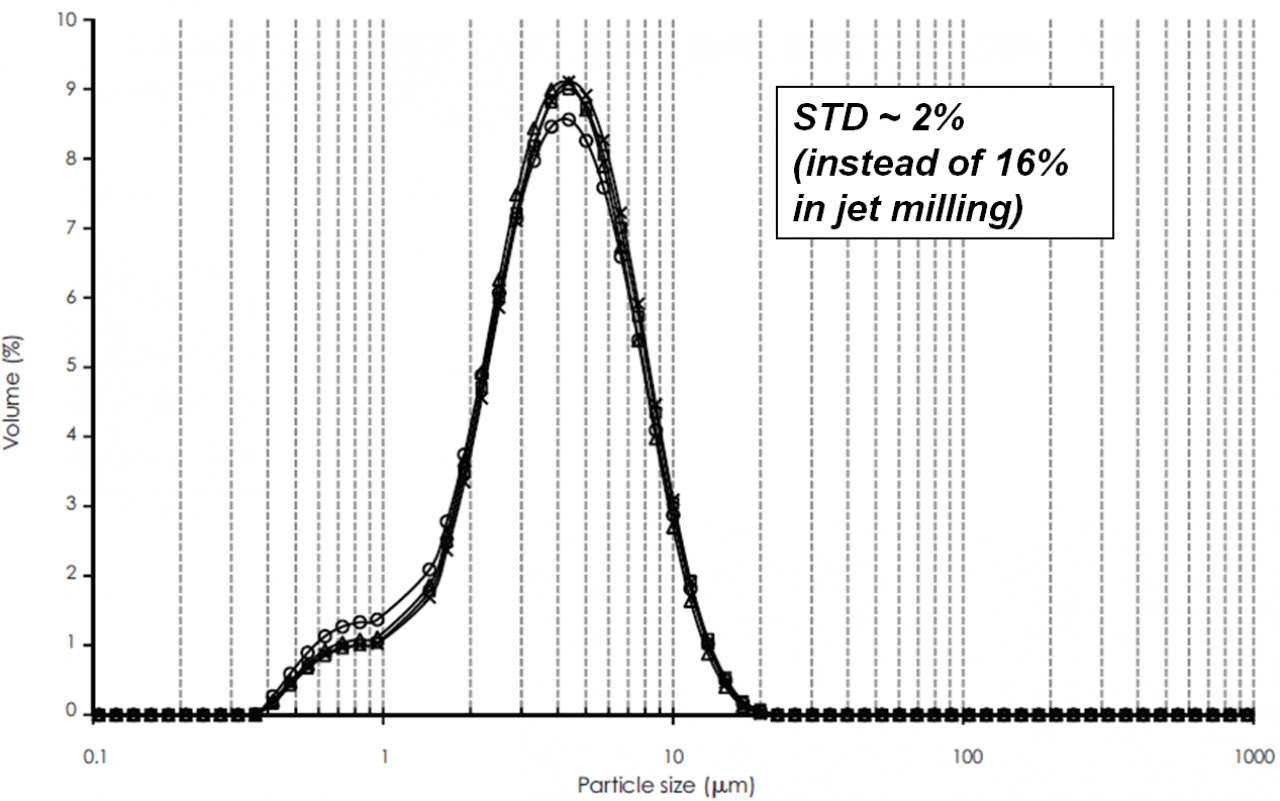
Hovione can fine tune the particle size distribution (PSD) of the API with unprecedented control, significantly exceeding what can typically be achieved by jet milling. Hovione allows its customer to target challenging DV50 and very tight spans with demonstrated consistency between batches and scales. The reduction in variability of API particle sizes distribution simplifies the formulation development effort and lends itself to improved product performance. Customized Inhalation grades with the desired particle size can be manufactured to meet customer requisites.
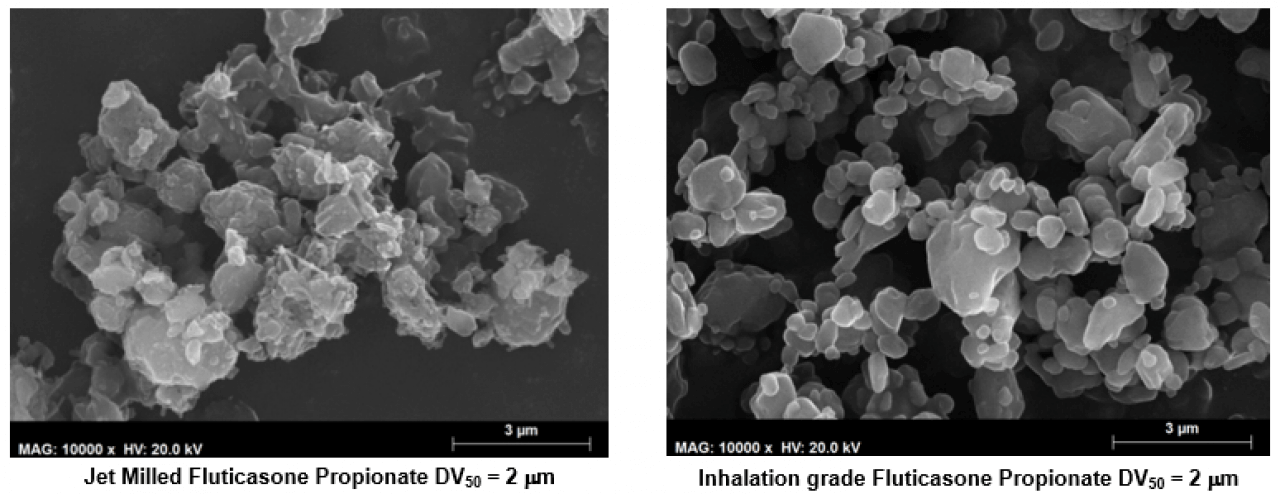
Hovione customized inhalation grade APIs are designed at the particle level. They have very little or no amorphous content with the possibility to tune surface properties and morphology. Comparative studies using Scanning Electron Microscopy (SEM) show the morphology differences between fluticasone propionate inhalation grade and fluticasone propionate obtained by jet milling.
Our APIs present:
- Scale-flexible processes
- Capability for Dv50 < 5 μm & Dv90 < 10 μm particle sizes
- Narrow span materials
- High reproducibility (PSD variability within ± 0.3 μm)
- Tunable surface properties & morphology
- Commercial batch sizes suitable to diverse needs
In addition, Hovione is ready to discuss your small and macro-molecules especially for delivery via the inhalation or nasal routes.
Hovione inhalation grades API current offering:
- Fluticasone Furoate
- Fluticasone Propionate
- Vilanterol Trifenatate
- Umeclidinium Bromide
- Mometasone Furoate
- Salmeterol Xinafoate
Please feel free to contact us if you have other inhalation or nasal APIs of interest.
Hovione Off Patent API
Impeccable chemical purity and unique product performance.
/products-and-services/patent-api-products/api-portfolio