Devices
Hovione offers simple, patented and globally competitive dry powder inhalers – Disposable, Capsule, Blister and Large Dose DPIs – which are available for fully integrated turn-key development with formulation and for licensing.
Device technology innovators
Hovione holds over 20 years of inhaler technology knowledge and marketing experience on innovative inhalation devices. We have established partnerships towards bringing affordable world-class inhalation medicines to patients. Hovione has developed the first market approved disposable dry powder inhaler for influenza treatment in Japan, which has become a notable success story resulting in millions of patients being treated every year with our innovative inhaler technology.
Full range of DPIs: available off-the-shelf and with long patent life
Hovione offers a complete portfolio of platforms suitable for both innovator and generic developments. All our DPIs are simple to use, innovative and built from few plastic parts, which makes them cost-effective at global scale and reduces development cost and risk. They are available off-the-shelf, and also for integrated development with formulation and for licensing.
| Sunriser® - High Performance, High-Dose Capsule DPI | |
|---|---|

|
|
| TwinCaps® – World’s largest selling disposable DPI | |
|---|---|

|
|
| Large Dose DPIs – Disposable DPIs for high dose drug delivery | |
|---|---|
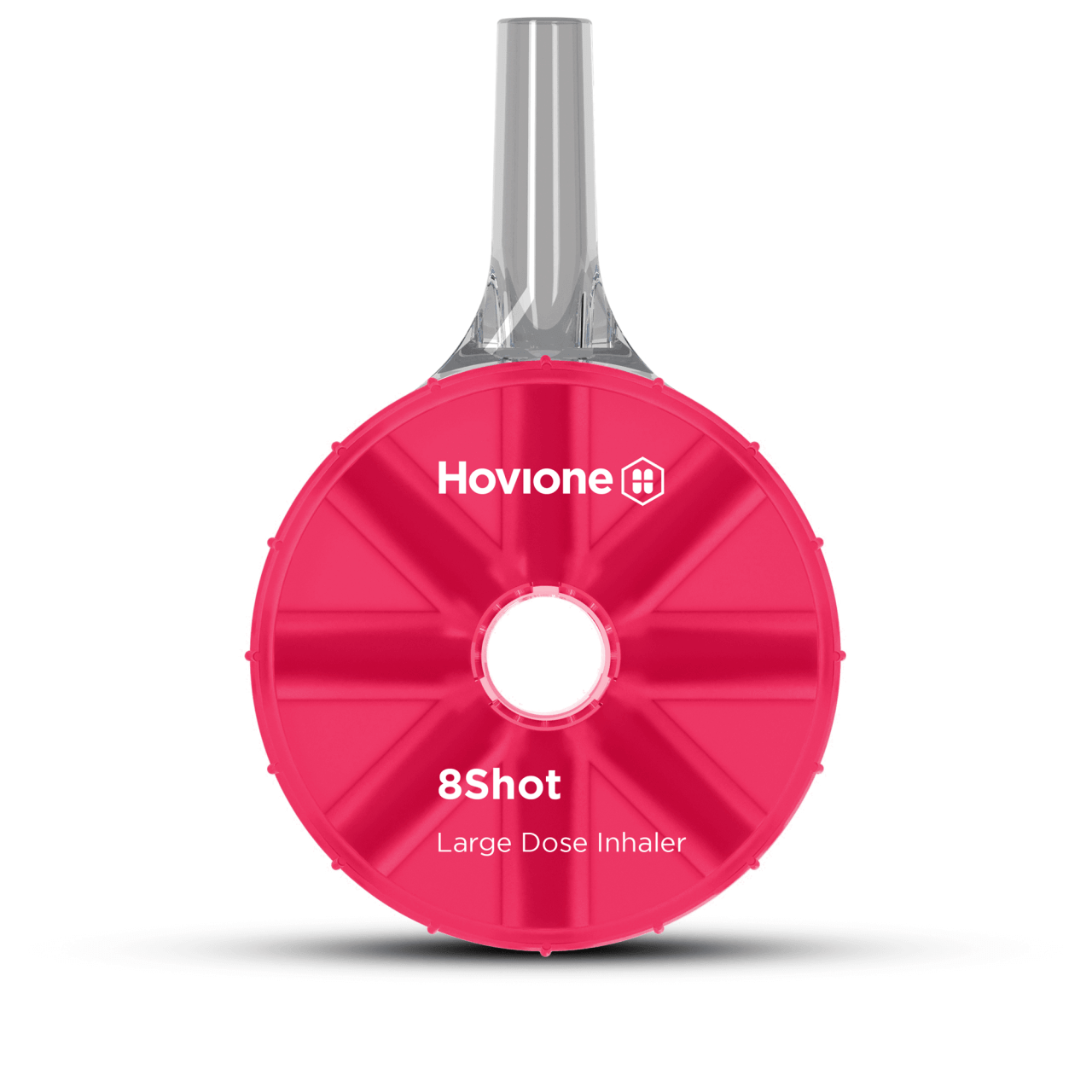
|
|
| PowdAir® – Easy-to-use, multi-use capsule DPI | |
|---|---|

|
|
| Papillon – Ultra-affordable, multi-use blister DPI | |
|---|---|
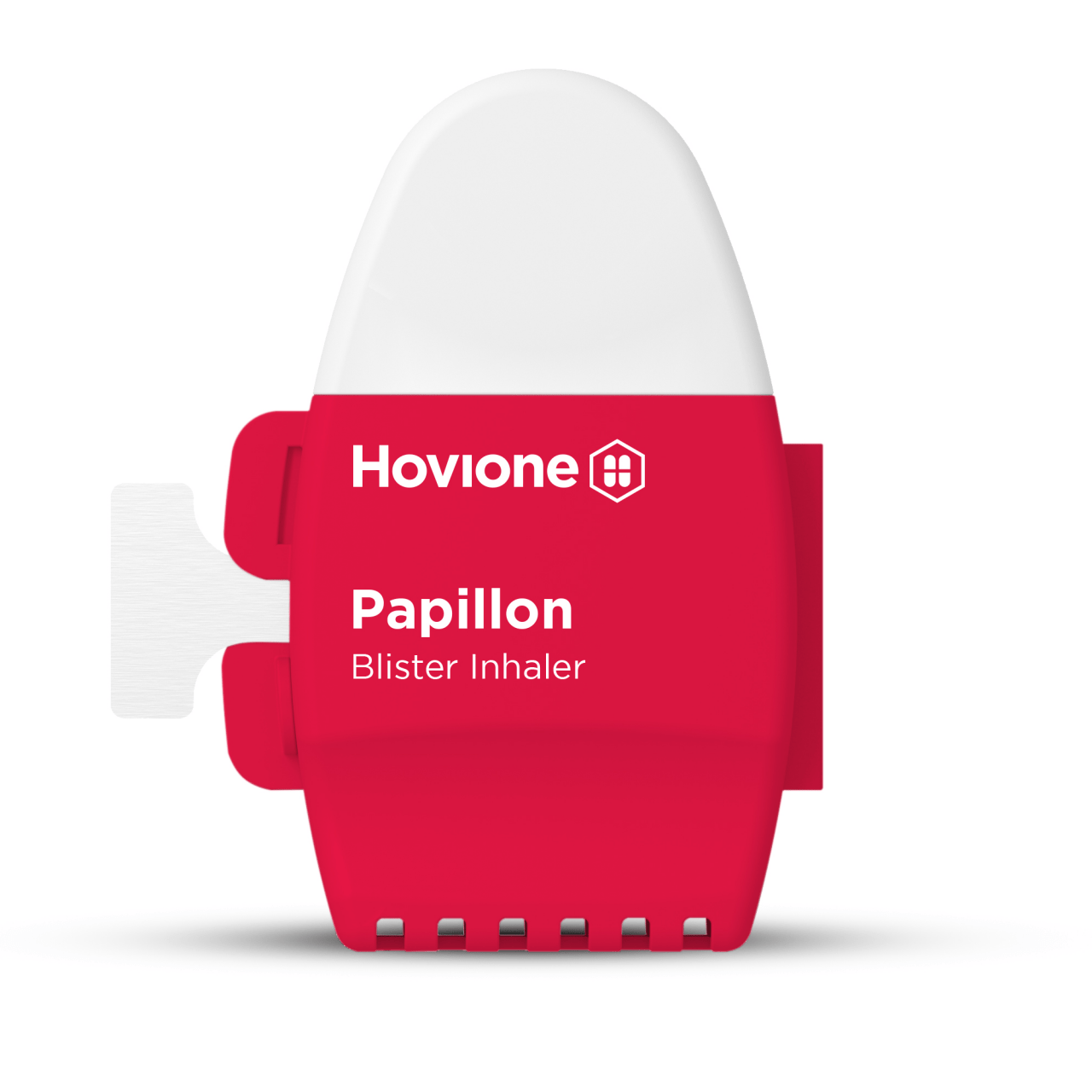
|
|
Fully Integrated Solutions for Inhalation
An Inhaled product development requires successful integration of Particle, Formulation, Device and Advanced Analytical Characterization of the Drug Product. Under one single roof, Hovione provides customers the knowledge and experience in technology selection and finding solutions to maximize the success probability and meet the needs of the disease, the patient, as well as keeping within the development program’s deadlines and budget. We are equipped to address all challenges regarding small molecules and biologics, to supply different manufacturing scales and to provide integrated solutions from feasibility to commercial.
Contact our experts today to bring your Inhaled medicine to patients
Contact Us
Please contact us if you have inquiries about our offering
https://go.hovione.com/l/47122/2014-08-06/9grc