Filling
Fully-integrated Filling for your device needs.
The nature of our work gives us the best of both worlds: we support and share the challenges of pharmaceutical development and all the science behind it while working in a controlled environment as a GMP certified manufacturing facility, all in one site.
State of the art manufacturing plant
Our infrastructure, technology and equipment enable us to work with highly potent products, ensuring high containment and safety. At the same time, our facility which incorporates built-in engineering solutions and safeguards, allows multiple product manufacturing areas with a low risk of cross contamination. We have a portfolio of equipment including state-of-the-art technologies, from drum to dosator filling, finely tuned for filling diverse types of powders into capsules or devices. Our advanced equipment is designed to meet the unique demands of inhalation dry powder filling.
We support and supply at different manufacturing scales, integrating respiratory drug delivery solutions from early clinical development all the way to commercial.
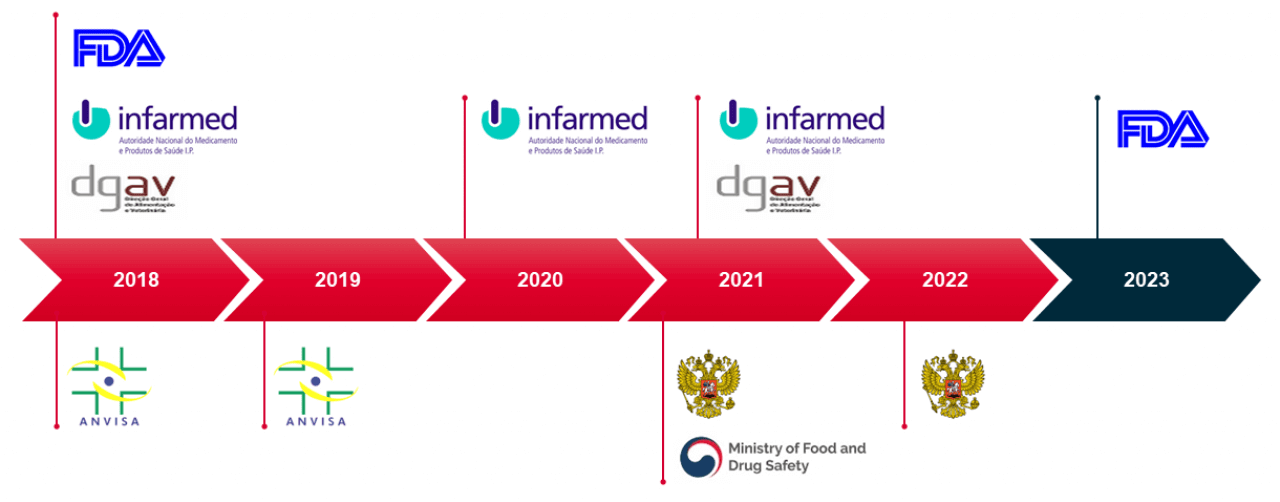
Our facilities are also inspected and approved by all major regulatory authorities and audited by dozens of clients every year.
Working with Hovione allows partners to take advantage of GMP facilities operated by highly skilled and responsive staff.
- High and low shear mixing
- Precision capsule filling 100% weight check (up to 55000 caps/hour), through Auger, Dosator or Drum filling technologies.
Our motto is “Delivering Solutions, Every Breath of the Way” meaning we offer Science that Solves throughout the life-cycle of your project delivering a one site fully integrated solution that reduces risk and cost, streamlines our experience and leadership while fostering collaboration.
Contact our experts today.
Our unique one-site set up facilitates collaboration between functions, providing opportunity for fresh thinking and creative approaches to your complex problems.
Learn more with the following article by Hovione's experts:
