Analytical Characterization
Hovione advanced characterization capabilities of inhalation and nasal products is crucial to determine their safety and efficacy.
Hovione has capabilities to perform in vitro testing of dry powder inhalers and nasal formulations, from method development and optimization to transfer or validation. Equipped with temperature and humidity- controlled rooms, analytical support can be given to projects in different development stages.
In addition to chemical and physical characterization, QC and stability of inhalation drug products testing includes specific techniques such as Delivered Dose Uniformity (UDD) and Aerodynamic Particle Size Distribution (aPSD) by cascade impaction (using NGI and ACI). During method development the use of abbreviated cascade impaction equipment, such as the FSI, for fast screening of different formulations can also be performed. Analytical method development follows a systematic approach and analytical quality by design (AQbD) methodologies.
Our analytical technologies can support our customers in getting quickly into clinic stages while maximizing chances of success
- Cascade impaction (NGI, ACI, FSI) coupled with biorelevant tools for accurate performance characterization (breathing simulator, Alberta Idealized Throat and Realistic Throat Model)
- Semi-automation tools for inhaler testing (NGI assistant)
- Benchtop environmental chamber
- Biorelevant dissolution for inhalation products (DissolviT and paddle over disk)
- Cascade impaction using AINI Alberta Idealized Nasal Inlet) nasal cast for nasal powders
- NGI cooler to support testing at controlled temperature environment
- Particle surface analysis: Raman imaging coupled with atomic force microscopy, specific surface area (by BET) and specific surface energy (by inverse gas chromatography)
- Laser diffraction for evaluation of particle size and characterize powder dispersibility
- Physical characterization (DSC, TGA, DVS, XRPD, solution calorimetry, SEM) and Amorphous content determination
- Powder rheology (BD/TD and shear cell)
Standard aerodynamic particle size distribution gives a narrow perspective on in vivo performance. Many other factors may determine the API fate: deposition pattern, dissolution rate, drug particle properties and physiological factors.
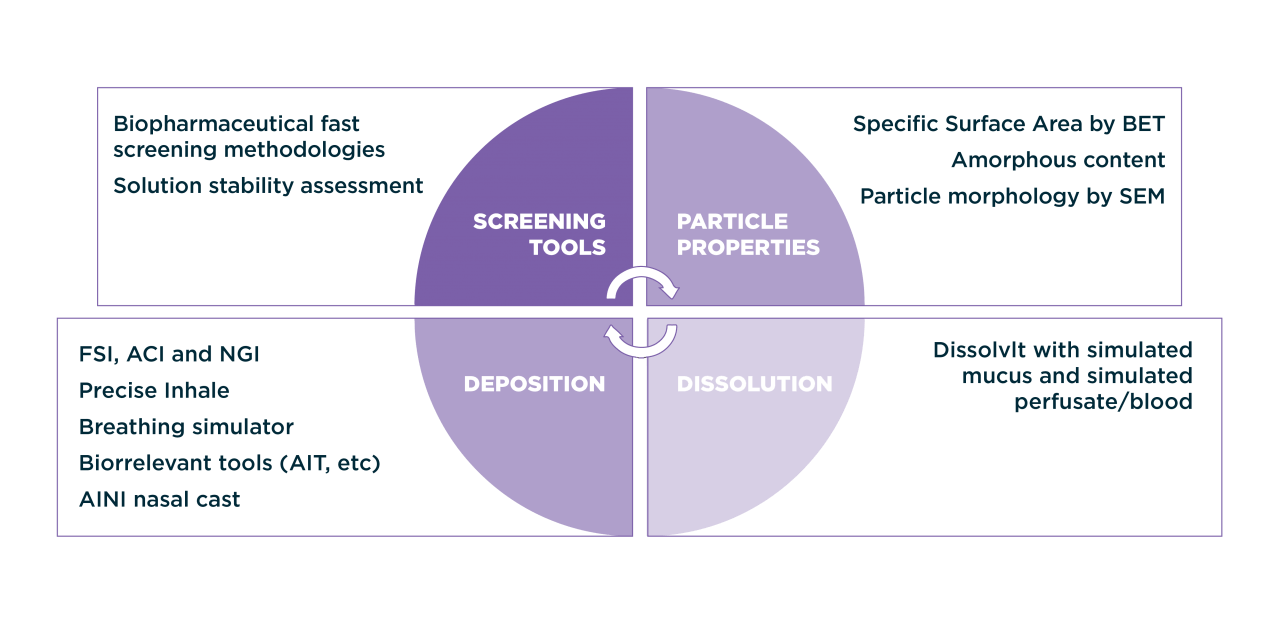
Hovione characterization capabilities
To cope with the need of development of clinically relevant in vitro tools for prediction of in vivo regional drug deposition and dissolution we have the capability to perform biorelevant dissolution testing with DissolvIt® coupled with PreciseInhale® into the advanced analytical characterization of dry powder inhalers (DPIs).
DissolvIt®
The DissolvIt® (from Inhalation Sciences) is an in vitro biorelevant tool to monitor absorption and dissolution of inhalable particles. It is used in combination with PreciseInhale® that will generate a gentle and highly controllable stream of aerosol powder to collect the inhalable fraction.
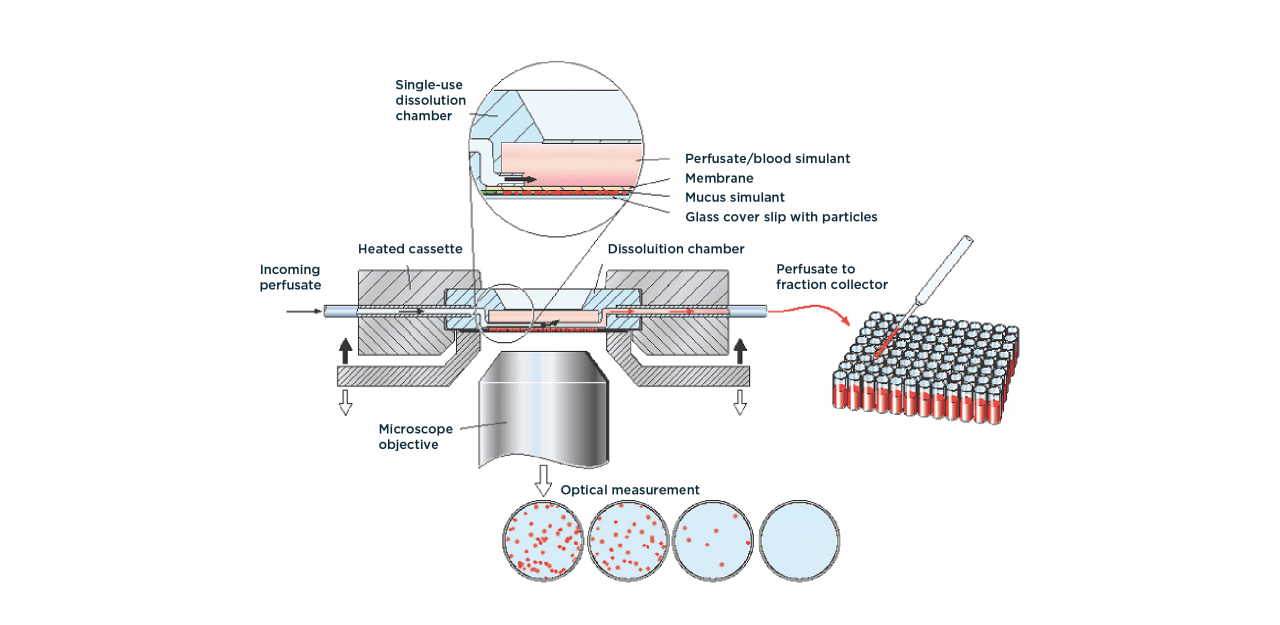
The inhaled powder is then placed in the dissolution and absorption module that uses an artificial air-blood barriers at physiological conditions to generate IVIVC data.
Applicability:
- first stages of formulation development, as the formulation strategy is defined (eg. Carrier-based vs carrier free)
- additional discrimination tool for formulation selection
We understand the complexity of developing highly sophisticated inhalation formulations, from the particle engineering stage to the device design, characterization and performance prediction. With our characterization tools we offer a faster path to the clinic driven by a strong scientific knowledge.
Contact our experts today.
Our team is ready to deliver solutions every breath of the way to help you fully develop your next carrier-free formulation.
Learn more with the following article by Hovione's experts:
