Hovione Macau
Hovione PharmaScience Ltd
Estrada Coronel Nicolau de Mesquita
Taipa
Macau
Tel: +853 2882 7544
Fax: +853 2882 7714
e-mail: infohm@hovione.com
DUNS Nr: 854974342
Location: 22°09'28.62" N 113°33'40.58" E

Oops! Your browser version is not supported by our website.
Please use these following browsers to view this page:
The site was first approved by the FDA in 1982, and is regularly inspected.
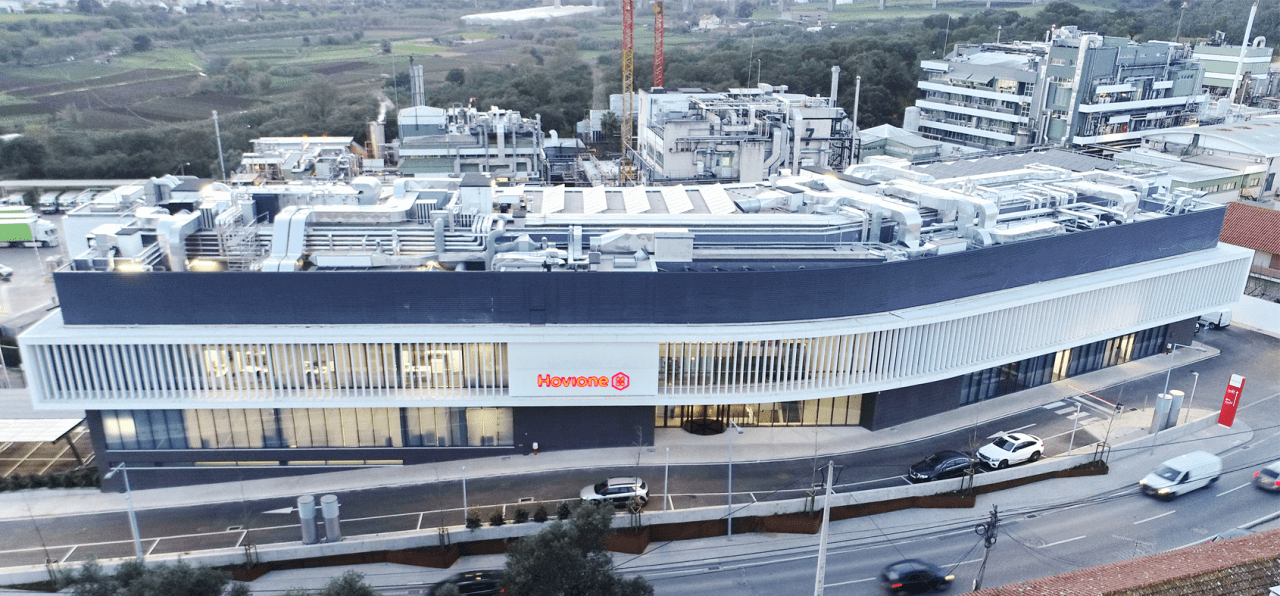
Hovione Loures has been operational since 1969. The site has grown continually as upgraded facilities have been added. The plant was first approved by the FDA in 1982, and is regularly inspected.
The site has an extensive capacity for drug substance manufacturing and particle engineering. Hovione Loures can support the development, piloting and full commercialization of your drug substance and drug product intermediate based on an available capacity of 430 cubic meters of vessels, ranging from 50 to 14,000lt, complemented with a full range of spray dryers, from lab scale to industrial sizes.
The site houses a large and well qualified Process Development and Analytical Development team in the areas of Drug Substance, Particle Engineering and Drug Product.
Areas of Focused Expertise
The site has world class capabilities and physical assets in the following areas:
For details about production equipment – click here
Operations
Certifications
The site has an Health, Safety and Environment Management System in accordance with OHSAS18001 and ISO14001. The system is periodically audited and reviewed and is ISO14001 and OHSAS18001 certified.
The site is a certified Authorized Economic Operator.
Hovione Farmaciência S.A. is a member of the BCSD Portugal which reflects our commitment to work for a more sustainable business.
Hovione Farmaciência S.A.
Sete Casas
2674-506 Loures
Portugal
Tel.: +351 21 982 9000
Fax: +351 21 982 9388
Email: contact@hovione.com
DUNS Nr: 449818434
Location: 38°50'33.526" N 9°10'42.240" W
Hovione PharmaScience Ltd
Estrada Coronel Nicolau de Mesquita
Taipa
Macau
Tel: +853 2882 7544
Fax: +853 2882 7714
e-mail: infohm@hovione.com
DUNS Nr: 854974342
Location: 22°09'28.62" N 113°33'40.58" E
Hovione Ltd
Loughbeg
Ringaskiddy
Co. Cork
Ireland
P43 DY23
Tel.: +353 21 451 2856
Fax: +353 21 437 8697
Email: contact@hovione.com
DUNS Nr: 985003840
Location: 51°49'20-01" N 8°18'28.23" W
Hovione LLC
40 Lake Drive
East Windsor, NJ 08520
USA
Tel.: +1 609 918 2600
Email: contact@hovione.com
Location: 40°15'50.01" N 74°29'41.16" W
Hovione Holding Asia Limited
1006 Room, Spaces 8
Queen´s Road East
Admiralty
Hong Kong
Tel.: +852 3190 3700
Hovione Holding Asia Limited
C/o WeWork, 2nd floor,
Oberoi Commerz - II, 1,
Off Western Express Highway,
Oberoi Garden City, Goregaon East,
Mumbai, Maharashtra 400063
India
Tel.: +919 561 830 188
Hovione Japan
GRAND GREEN OSAKA North BLDG, JAM BASE 4F, 6-38 Ofuka-cho, Kita-ku, Osaka-shi, Osaka
Japan
Tel: +81 6 6131 4619
www.hovione.co.jp
Hovione Holding AG
Lindenstrasse 14
6340 Baar
Switzerland
Hovione LLC
50 Millstone Road
Building 300, Suite 100
East Windsor, NJ 08520
USA
Email: contact@hovione.com
Location: 40°17'13.7"N 74°33'33.6"W
Hovione (Shanghai) Pharma Technology Limited
Room 2206, 22/F, 710 Dongfang Road, China (Shanghai) Pilot Free Trade Zone, PRC, 200122
Tel.: +86 21 62120210
contact@hovione.com
www.hovione.com.cn
Hovione (Shanghai) Pharma Technology Limited Beijing Branch
Room 1003-1, 10/F, Sanyuan NEO Xinjing Building, Building 18, No. 5 Shuguang Xili, Chaoyang District, Beijing, China
Tel.: +86-18500027185
Tel.: +86-19931153253
contact@hovione.com
www.hovione.com.cn
Hovione Farmaciência S.A.
Estrada do Paço do Lumiar,
Campus do Lumiar, Edifício R
1649-038 Lisboa
Portugal
Tel: +351 21 982 9000
Fax: +351 21 982 9388
E-mail: contact@hovione.com
DUNS Number: 338285630
Location: 38°46'21.72" N -9°10'49.1874" W