Lisbon, December 14, 2022, Hovione and GEA today announced a strategic collaboration to advance Continuous Tableting. This partnership stemmed from a successful customer-supplier relationship spanning several years. It combines GEA´s engineering expertise with Hovione´s development and manufacturing experience and both parties commit to partner to accelerate the adoption of Continuous Tableting technology.
The advantages of continuous over batch manufacturing are well known. Continuous production systems allow for leaner and risk-reduced development paths, leaner supply chains, increased built-in quality, and in general, manufacturing processes of greater flexibility and reduced complexity. In the context of this strategic collaboration, Hovione and GEA will combine their strengths to further advance the technology and contribute to the establishment of new standards and new levels of market acceptance.
“Hovione has a track record in industrializing and democratizing emerging pharmaceutical technologies, such as amorphous solid dispersions by spray drying, and turn them into dependable and scalable offerings that are available to all.” Says Jean-Luc Herbeaux, CEO of Hovione. He adds “We have been committed to Continuous Tableting for the last 7 years and we are now ready to contribute decisively to the advancement of one of the most promising technologies in pharma manufacturing. This collaboration with GEA, gives us the opportunity to link up with a leading designer and supplier of Continuous Tableting equipment solutions and bring Continuous Tableting to the next levels of reliability, flexibility and adoption.”
Navin Lakhanpaul, Global Head Pharma-Solid Dosage, GEA, adds: “As a leading supplier of process technology, GEA has established itself as a trusted solution provider in the pharmaceutical industry, and as a pioneer in the development of Continuous Tableting technology.” He adds “Our relationship with Hovione over many years has clearly demonstrated the value in further collaborating and developing synergies in our respective areas of expertise. We share a common objective of enabling the wider use of Continuous Tableting in the Pharmaceutical Industry, and firmly believe that our partnership with Hovione will accelerate the growth of this exciting technology."
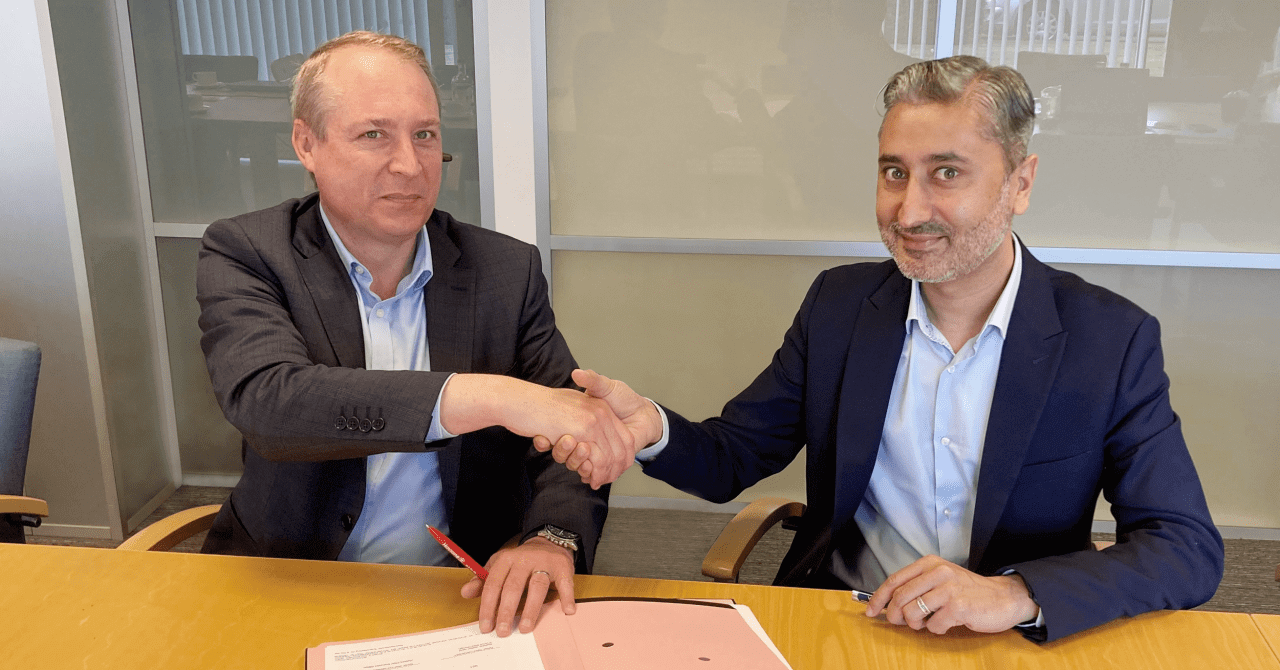
Navin Lakhanpaul, Global Head Pharma-Solid Dosage, GEA (right) and Jean-Luc Herbeaux, CEO of Hovione (left), signed the agreement for close cooperation between the two companies. (Photo: GEA)
About Hovione: Hovione is an international company with over 60 years of experience as a Contract Development and Manufacturing Organization (CDMO) and is currently a fully integrated supplier offering services for drug substance, drug product intermediate and drug product. With four FDA inspected sites in the USA, China, Ireland, and Portugal and development laboratories in Lisbon, Portugal and New Jersey, USA, the company provides branded pharmaceutical customers services for the development and compliant manufacture of innovative drugs including highly potent compounds and customized product solutions across the entire drug life cycle. In the inhalation area, Hovione offers a complete range of and services, from API, formulation development and devices. Hovione was the first Chemical/ Pharmaceutical Company to became a Certified B Corp, is a member of Rx-360, EFCG and participates actively in the industry standard setting process.
About GEA: GEA is one of the world's largest suppliers of systems and components to the food, beverage and pharmaceutical industries. The international technology¬ group, founded in 1881, focuses on machinery and plants, as well as advanced process technology, components, and comprehensive services. With more than 18,000 employees working across five divisions and 62 countries, the group generated revenues of more than EUR 4.7 billion in fiscal year 2021. GEA plants, processes, components and services enhance the efficiency and sustainability of production processes across the globe. They contribute significantly to the reduction of CO2 emissions, plastic usage and food waste. In doing so, GEA makes a key contribution toward a sustainable future, in line with the company’s purpose: "Engineering for a better world".
GEA is listed in the German MDAX and the STOXX® Europe 600 Index and is also among the companies comprising the DAX 50 ESG and MSCI Global Sustainability Indices.
Learn more about Continuous Tableting at Hovione