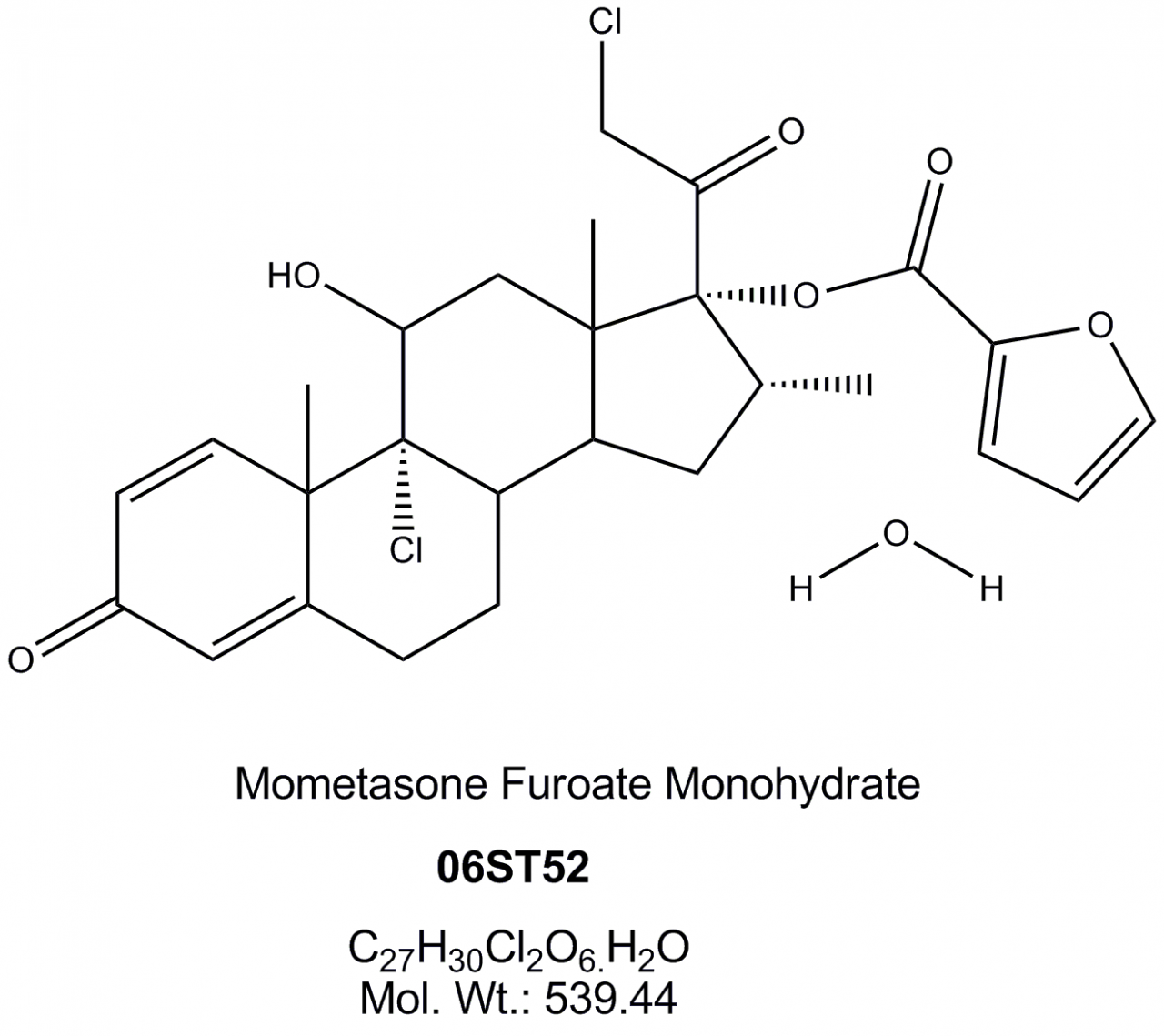
Mometasone Furoate Monohydrate
- Inhalation
- Hovione Loures
- Corticosteroids
- Inhalation
- Nasal
- Allergic rhinitis
- Asthma
Hovione can customize Mometasone Monohydrate API for nasal suspensions or inhalation based on its experience in particle size reduction technologies with tailored made, highly reproducible particle size distributions.
High crystalline purity, low amorphous content with prolonged stability are also part of our offering.
Hovione Mometasone Monohydrate API is available since 2008 and is approved in generic applications.
This is not to be construed as a representation of non-infringement or as an offer to sell in those countries where such would constitute an infringement of third parties' patent rights.
Contact Us
Please contact us if you have inquiries about our offering
https://go.hovione.com/l/47122/2014-08-06/9grc