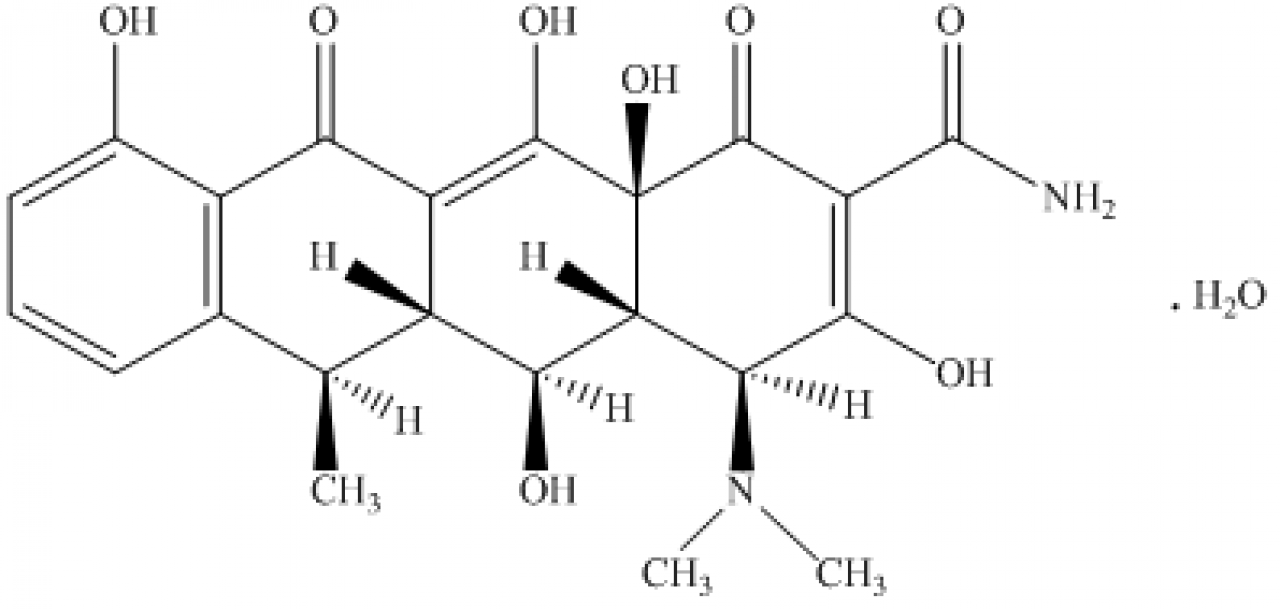
Doxycycline monohydrate
- Micronized
- Sieved
- Hovione Loures
- Tetracycline Antibiotic
- Oral
- Acne
- Anthrax exposure
- Malaria prevention
- Other bacterial infections
Market leaders supplying Doxycycline Monohydrate in regulated markets such as the US, Europe and Japan from multiple manufacturing sites since 1982.
Hovione offers an API with a high grade quality manufactured in dedicated facilities for human applications. Customizable particle size distribution to fit end application and formulation requirements.
Hovione Doxycycline Monohydrate is approved in branded, generic and 505b2 applications.
This is not to be construed as a representation of non-infringement or as an offer to sell in those countries where such would constitute an infringement of third parties' patent rights.
Contact Us
Please contact us if you have inquiries about our offering
https://go.hovione.com/l/47122/2014-08-06/9grc