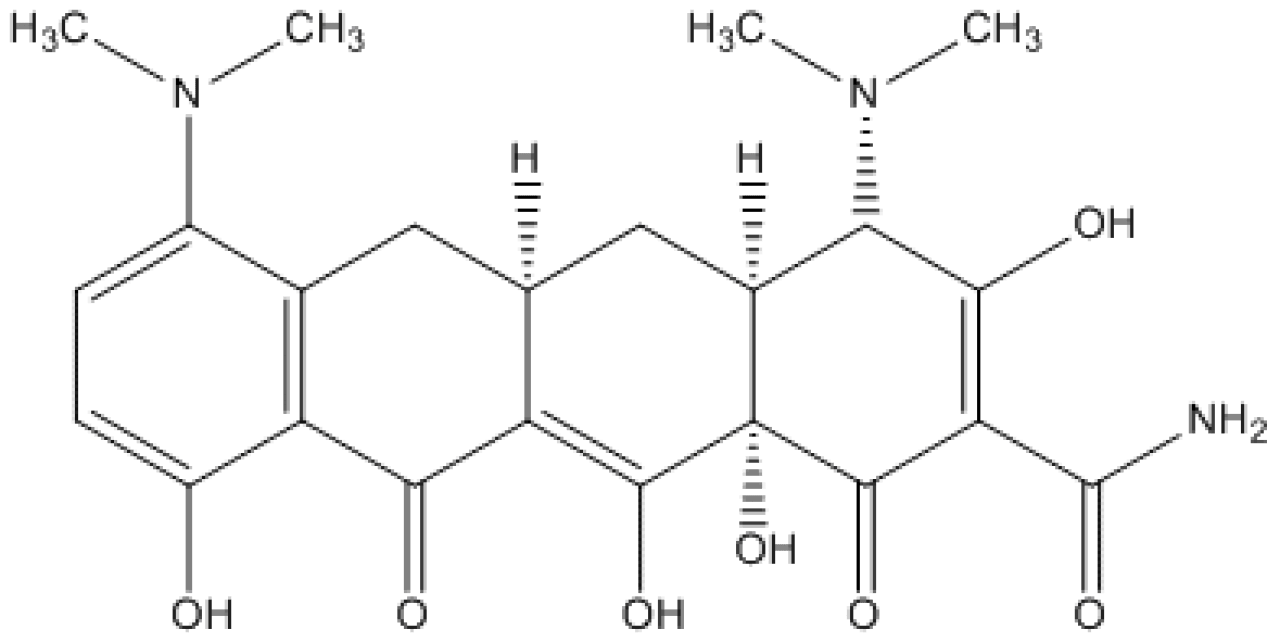
Minocycline base
API Product
Product Status:
Commercial
Available Grades:
- Micronized
Regulatory Status:
US DMF
Production Sites:
- Hovione Loures
Product Type:
- Tetracycline Antibiotic
CAS Number:
10118-90-8
Modes of Application:
- Oral
- Injectable
- Anti-Infectives
Common Indications:
- Acute Acne
Last Inspection:
FDA May 2018
Hovione offers stable cGMP Minocycline Base, crystalline material for both unmicronised and micronised grades.
The product is manufactured at industrial scale at high purity and stability.
Hovione has IP on Minocycline Base that can help you extend your exclusivity period in case you are developing new formulations or looking to new indications.
This is not to be construed as a representation of non-infringement or as an offer to sell in those countries where such would constitute an infringement of third parties’ patent rights.
Highlight
Contact Us
Please contact us if you have inquiries about our offering
https://go.hovione.com/l/47122/2014-08-06/9grc